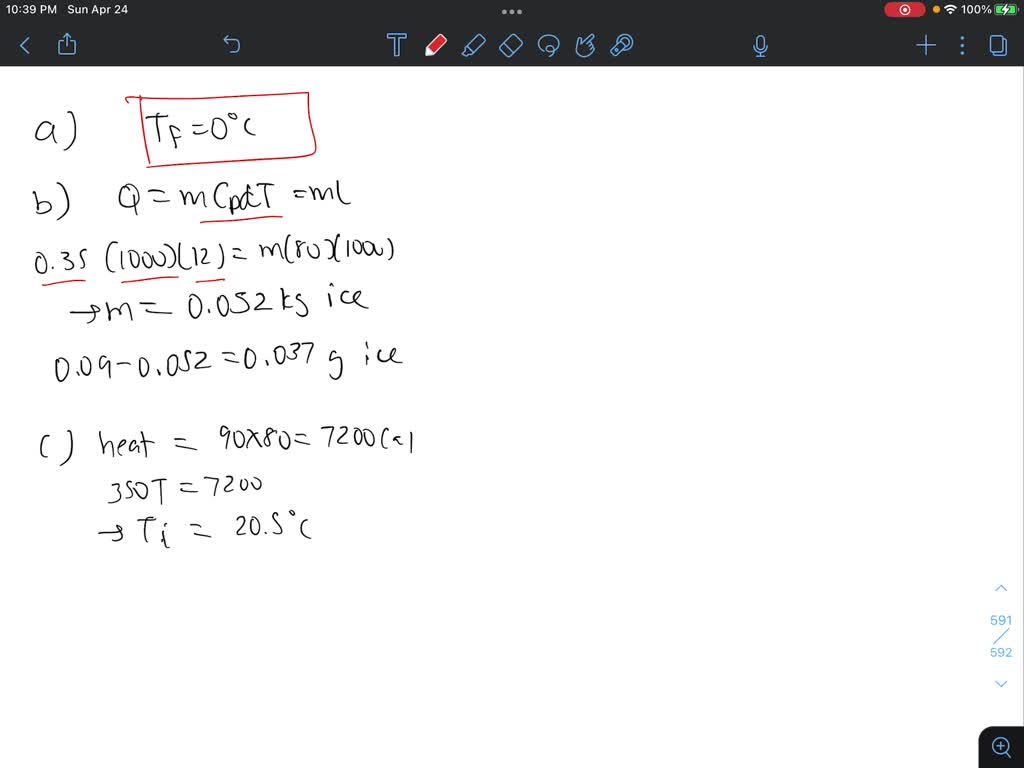
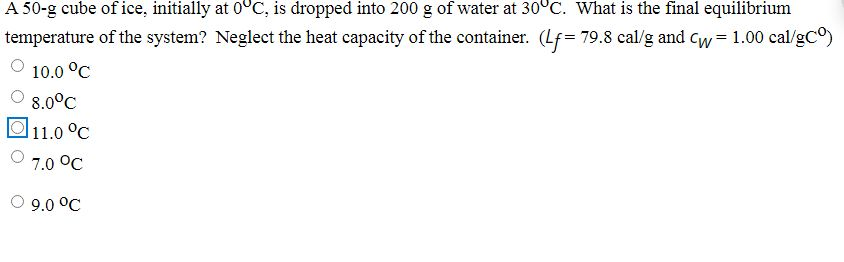Solved IP A 35−g ice cube at 0.0∘C is added to 120 g of
By A Mystery Man Writer
Last updated 21 Sept 2024

Answer to Solved IP A 35−g ice cube at 0.0∘C is added to 120 g of
Energies November-1 2021 - Browse Articles
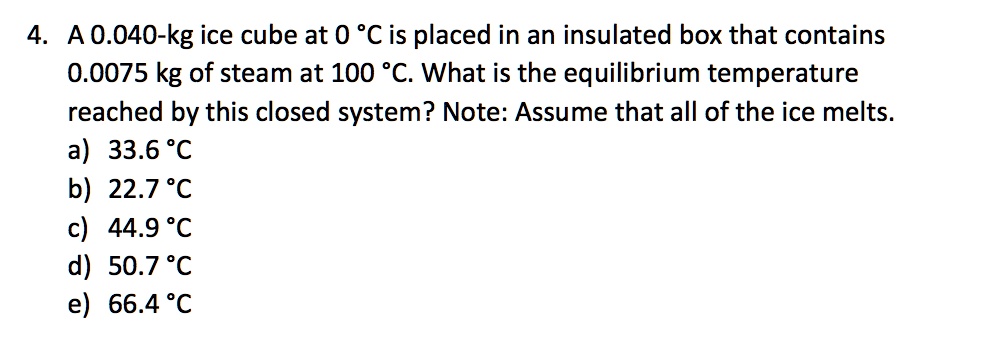
SOLVED: 4. A 0.040-kg ice cube at 0 *C is placed in an insulated
REVIEWER FOR ChE COMPRE 07082018, PDF, Chemistry
How much energy is required to change a 35 g ice cube from ice at -15 degrees C to steam at 120 degrees C? - Quora
Chem PDF, PDF, Mole (Unit)
Solved A 50-g cube of ice, initially at 0°C, is dropped into
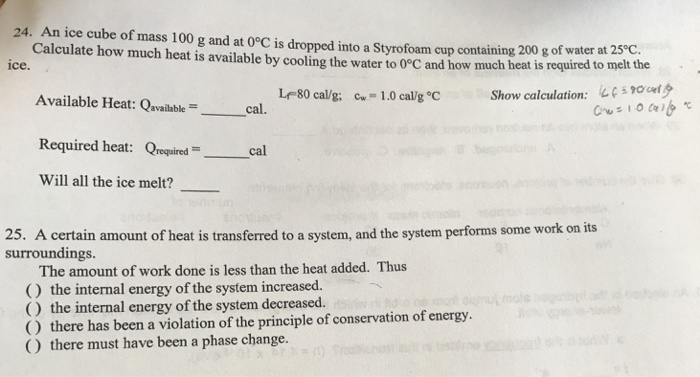
Solved An ice cube of mass 100 g and at 0 degree C is
19-20) A 35-g ice cube at its melting point is dropped into an
Solved How much energy is required to change a 35 g ice cube
SOLVED: A 9.0×10^(-2)-kg ice cube at 0.0 °C is dropped into a Styrofoam cup holding 0.35 kg of water at 12 °C. Part A: Find the final temperature of the system. Assume
Recommended for you
 Conversion chart Baking measurements, Cooking measurements, Baking conversions14 Jul 2023
Conversion chart Baking measurements, Cooking measurements, Baking conversions14 Jul 2023 Blue / Clear Plastic 35 Gram Measuring Spoon at best price in Vadodara14 Jul 2023
Blue / Clear Plastic 35 Gram Measuring Spoon at best price in Vadodara14 Jul 2023 Cup Rubber Tactile Linear 35g Compatible For Topre DES Leopold Novotouch HHKB Realforce Capacitive Keyboard14 Jul 2023
Cup Rubber Tactile Linear 35g Compatible For Topre DES Leopold Novotouch HHKB Realforce Capacitive Keyboard14 Jul 2023- Solved Problem 3 (Phase changes) A 35 g ice cube at 0°C is14 Jul 2023
- Shockinglydelicious - Cups to gramshow many sticks of butter in14 Jul 2023
) Buy Tata Care Rejuvenate Tea Bags 35 g (Pack of 25) Online at Best14 Jul 2023
Buy Tata Care Rejuvenate Tea Bags 35 g (Pack of 25) Online at Best14 Jul 2023 Cup Rubber Tactile Linear 35g Compatible For Topre Des Leopold14 Jul 2023
Cup Rubber Tactile Linear 35g Compatible For Topre Des Leopold14 Jul 2023 Gifts For Teachers – All For Her Gifts14 Jul 2023
Gifts For Teachers – All For Her Gifts14 Jul 2023 Are we relying too much on snack foods? - Let's Eat! Feeding Therapy14 Jul 2023
Are we relying too much on snack foods? - Let's Eat! Feeding Therapy14 Jul 2023- JAPAN ORIGINAL CIAO Pon Churu Tuna Creamy Cup Cat Treat (35g x 2 Cups)14 Jul 2023
You may also like
 Blouses & Button-Down Shirts14 Jul 2023
Blouses & Button-Down Shirts14 Jul 2023 Gedy 7630-13 By Nameek's Bridge Polished Chrome Wall Mounted Towel Rack With 3 16 Inch Sliding Rails - TheBathOutlet14 Jul 2023
Gedy 7630-13 By Nameek's Bridge Polished Chrome Wall Mounted Towel Rack With 3 16 Inch Sliding Rails - TheBathOutlet14 Jul 2023 Free People FP One Adella Bralette Embroidered Lace - Depop14 Jul 2023
Free People FP One Adella Bralette Embroidered Lace - Depop14 Jul 2023 Pink Racer Back Sports Bra - TK Maxx UK14 Jul 2023
Pink Racer Back Sports Bra - TK Maxx UK14 Jul 2023 Winter Outfits 2023 Chic Crop Top Wide Leg Pants Outfit 2-piece14 Jul 2023
Winter Outfits 2023 Chic Crop Top Wide Leg Pants Outfit 2-piece14 Jul 2023- Women's Lace Demi Longline Bra - Auden™ Blue 38dd : Target14 Jul 2023
 Sweet Vintage Floral - Custom Linen Cotton Fabric, per 1/2 meter14 Jul 2023
Sweet Vintage Floral - Custom Linen Cotton Fabric, per 1/2 meter14 Jul 2023 Camisoles Women Petite Tank Tops For Women V Neck Silk Summer Satin Sleeveless Blouse Basic Camisole Shirts14 Jul 2023
Camisoles Women Petite Tank Tops For Women V Neck Silk Summer Satin Sleeveless Blouse Basic Camisole Shirts14 Jul 2023 Floral Lace Underwire Lingerie Set14 Jul 2023
Floral Lace Underwire Lingerie Set14 Jul 2023 Lululemon Hits $6 Billion in Sales for the First Time – Visual14 Jul 2023
Lululemon Hits $6 Billion in Sales for the First Time – Visual14 Jul 2023