The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n
By A Mystery Man Writer
Last updated 20 Sept 2024
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://www.vedantu.com/question-sets/a60a955d-f16d-432e-852e-8dcb41060ecf8392656688535846153.png)
The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n A.For the gas A, a=0 and its dependence
REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR
The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.
stackoverflowresearcher/archimedes.txt at master · MichalPaszkiewicz/stackoverflowresearcher · GitHub
The given graph represents the variations of compressibility factor Z=PV//nRT vs P for three rea
Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with
Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with
The graph of compressibility factor Z vs P for one mole of a real gas is shown in following diagram. The graph is plotted at a constant temperature 273 K. If the
Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with
Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with
The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `
For the gas C which is a typical real gas for which neither a nor b =0
stackoverflowresearcher/archimedes.txt at master · MichalPaszkiewicz/stackoverflowresearcher · GitHub
Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with
Yucation The given graph represent the variations of Z (compressibility factor = pV) v/s p three nRT real gases, A, B and C. Identify the incorrect statement. p(atm) - A. For the
Recommended for you
- At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is14 Jul 2023
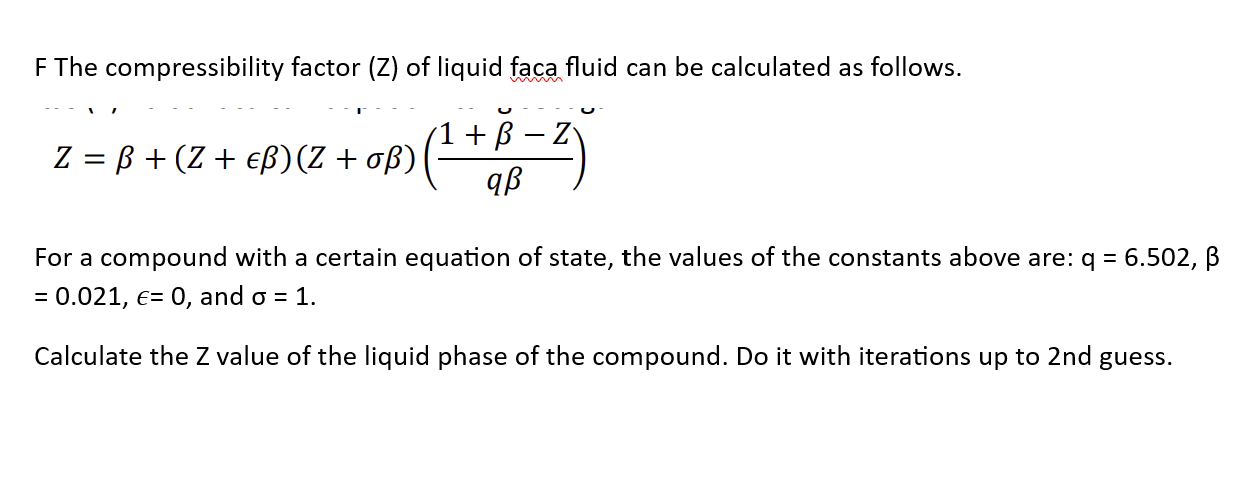
- Solved F The compressibility factor ( Z ) of liquid faca14 Jul 2023
 COMPRESSIBILITY FACTOR14 Jul 2023
COMPRESSIBILITY FACTOR14 Jul 2023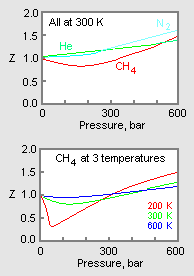 Compressibility factor (gases) - Knowino14 Jul 2023
Compressibility factor (gases) - Knowino14 Jul 2023 Compressibility Factor, Z, for Various Methods.14 Jul 2023
Compressibility Factor, Z, for Various Methods.14 Jul 2023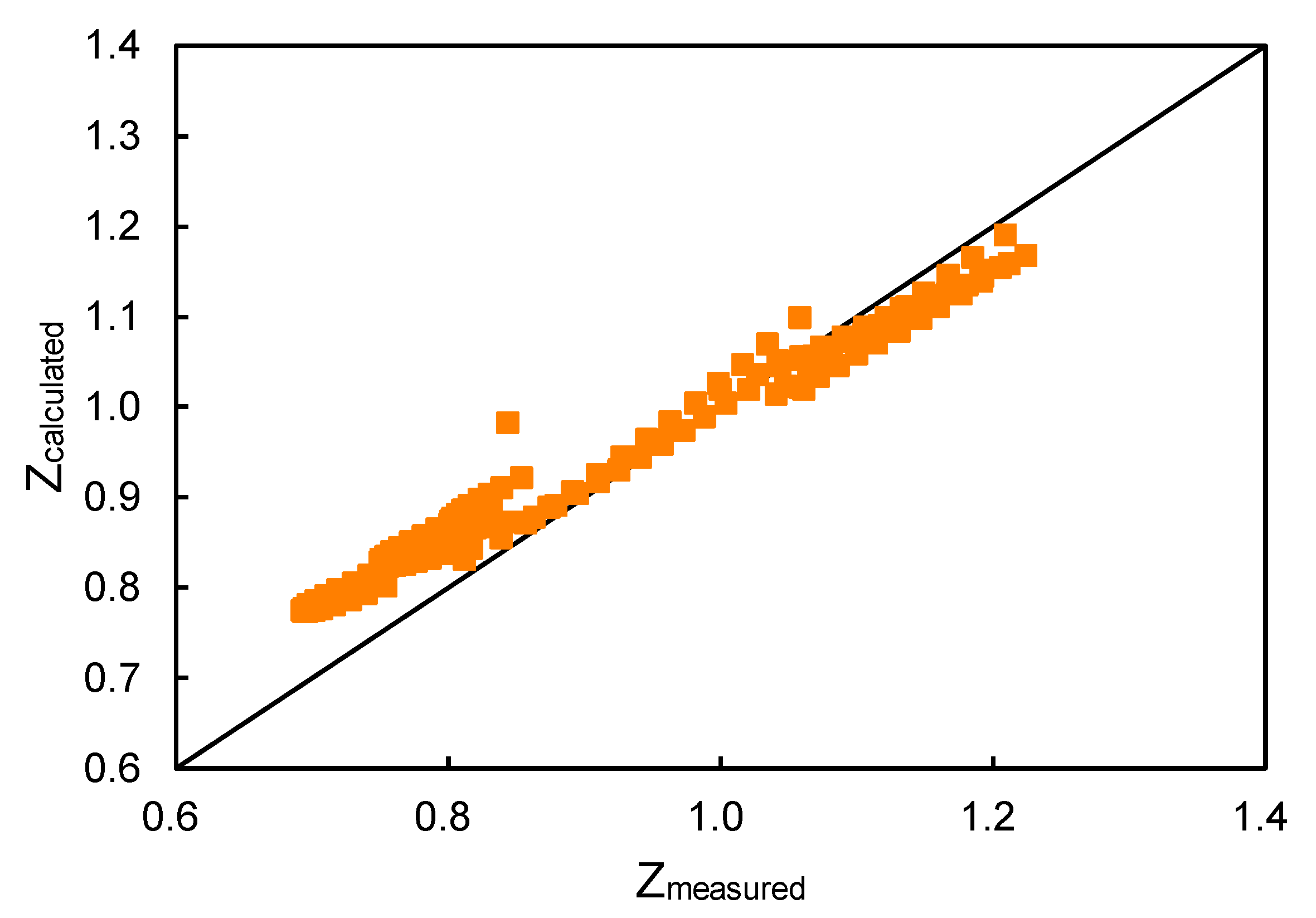 Energies, Free Full-Text14 Jul 2023
Energies, Free Full-Text14 Jul 2023![PDF] Compressibility Chart for Hydrogen and Inert Gases](https://d3i71xaburhd42.cloudfront.net/fff428eb21faf6b00221ccb7bf36c044c50d7e4a/1-Figure1-1.png) PDF] Compressibility Chart for Hydrogen and Inert Gases14 Jul 2023
PDF] Compressibility Chart for Hydrogen and Inert Gases14 Jul 2023 Calculate the Compressibility Factor 'z' for Hydrocarbon Gases • zFactor14 Jul 2023
Calculate the Compressibility Factor 'z' for Hydrocarbon Gases • zFactor14 Jul 2023 My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com14 Jul 2023
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created with Publitas.com14 Jul 2023- In the above figure, near the point B, compressibility factor Z is about..14 Jul 2023
You may also like
 34 E Strapless Bra Open Nipple Bra 36E Bra Multipack Shapewear14 Jul 2023
34 E Strapless Bra Open Nipple Bra 36E Bra Multipack Shapewear14 Jul 2023 Buy Zivame Lace Touch Full Sleeve Shaping Bodysuit Top-White at Rs14 Jul 2023
Buy Zivame Lace Touch Full Sleeve Shaping Bodysuit Top-White at Rs14 Jul 2023 9 Cheap & Clever Ways to Change Your Home Decor for Every Season14 Jul 2023
9 Cheap & Clever Ways to Change Your Home Decor for Every Season14 Jul 2023 Test This} Instant Breast Lifts by Bring It Up14 Jul 2023
Test This} Instant Breast Lifts by Bring It Up14 Jul 2023 Women's Metallic Support Sports Bra Top Criss-Cross Back Padded Yoga Bras Cami Crop Tops Clubwear14 Jul 2023
Women's Metallic Support Sports Bra Top Criss-Cross Back Padded Yoga Bras Cami Crop Tops Clubwear14 Jul 2023- Separatec Reviews Read Customer Service Reviews of www.separatec.com14 Jul 2023
 Whitney Simmons Net Worth: Details About Car, Career, , Income - SarkariResult14 Jul 2023
Whitney Simmons Net Worth: Details About Car, Career, , Income - SarkariResult14 Jul 2023 Shop the Stanley Quencher Deco Collection - PureWow14 Jul 2023
Shop the Stanley Quencher Deco Collection - PureWow14 Jul 2023 Nursing student invents cooling vest to help surgeons beat heat stress The Daily The Daily14 Jul 2023
Nursing student invents cooling vest to help surgeons beat heat stress The Daily The Daily14 Jul 2023 A Comfy, Elevated Casual Holiday Outfit - Classy Yet Trendy14 Jul 2023
A Comfy, Elevated Casual Holiday Outfit - Classy Yet Trendy14 Jul 2023
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](http://www.adichemistry.com/physical/gaseous/deviation/zvsp-graph1.png)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://s3.ap-south-1.amazonaws.com/byjus-media-delivery/videos/mpkgr-production-33da172a/rgfzcp/Search_QNA/220802/B11-B20/B13/20JEE12PHY01HN15WXX/Batch_1/20JEE12PHY01HN15W08/thumbs/4a46767a/480x360.jpg)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://i.ytimg.com/vi/RnF2u4BucTo/maxresdefault.jpg)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://i.ytimg.com/vi/7GVFRQDR0gc/hq720.jpg?sqp=-oaymwEhCK4FEIIDSFryq4qpAxMIARUAAAAAGAElAADIQj0AgKJD&rs=AOn4CLB2QIuNktt9kxmW9fPQK5b355wv_Q)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://i.ytimg.com/vi/T-bRGU0ZmFI/default.jpg)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://search-static.byjusweb.com/question-images/byjus/infinitestudent-images/ckeditor_assets/pictures/574788/original_SOM.Dai.jpg)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://i.ytimg.com/vi/T-bRGU0ZmFI/maxresdefault.jpg)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://i.ytimg.com/vi/_nHm42j-rrw/hq720.jpg?sqp=-oaymwEhCK4FEIIDSFryq4qpAxMIARUAAAAAGAElAADIQj0AgKJD&rs=AOn4CLCSGVRzOFRH9MjTcMeC_OMF-bUVlw)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://i.ytimg.com/vi/hYKtPIKpTok/hq720.jpg?sqp=-oaymwE7CK4FEIIDSFryq4qpAy0IARUAAAAAGAElAADIQj0AgKJD8AEB-AHUBoAC4AOKAgwIABABGH8gEygYMA8=&rs=AOn4CLBs8sRO4kBqTvmPgJFL8v7kXZ1PcA)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://static.doubtnut.com/ss/web-overlay-thumb/17366737.webp)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://i.ytimg.com/vi/oQZwgxCc7og/hq720.jpg?sqp=-oaymwEhCK4FEIIDSFryq4qpAxMIARUAAAAAGAElAADIQj0AgKJD&rs=AOn4CLBrqVI-yX_tn4XIVdZ6e6yUMkZIvw)
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://toppr-doubts-media.s3.amazonaws.com/images/3788251/98c0ccf1-8d03-4214-9e75-45ded4c5e0e0.jpg)