If an element gains an electron, will it form a positive ion or a
By A Mystery Man Writer
Last updated 21 Sept 2024
Negative Ion An atom on gaining an extra electron will acquire one unit of negative charge. An atom has equal number of protons and electrons. The total positive charge of protons balance out the total negative charge of electrons.
Chemical Bonding Review Worksheet, PDF, Ionic Bonding
B11 Lab Chemistry1.pdf - Chemical Bonding Review Worksheet 1. Which part of the atom is responsible for chemical bonding? Valence Electrons 2. What
B11 Lab Chemistry1.pdf - Chemical Bonding Review Worksheet 1. Which part of the atom is responsible for chemical bonding? Valence Electrons 2. What
B11 Lab Chemistry1.pdf - Chemical Bonding Review Worksheet 1. Which part of the atom is responsible for chemical bonding? Valence Electrons 2. What
Chemical Bonding Review WS 1 .pdf - Chemical Bonding Review Worksheet 1. Which part of the atom is responsible for chemical bonding? valence
SOLVED: 1. If an element gives away an electron, will it form a positive ion or negative ion? 2. If an element gains an electron, will it form a positive ion or
Chemical Bonding Review Worksheet, PDF, Ionic Bonding
Chemical Bonding Review Worksheet, PDF, Ionic Bonding
Chemical Bonding Review Worksheet, PDF, Ionic Bonding
Chemical Bonding Review Worksheet - Chemical Bonding Review Worksheet 1. Which part of the atom is responsible for chemical bonding? 2. What are
Chemical Bonding Review WS 1 .pdf
DOC) Chemical Bonding Review Worksheet - Murrieta … · Web viewThen write the chemical formula in the space provided. Ionic or covalent C Cl Formula _____ Ionic or covalent Mg Cl Formula
Chemical Bonding Review Worksheet
SOLVED: 1. If an element gives away an electron, will it form a positive ion or negative ion? 2. If an element gains an electron, will it form a positive ion or
Chemical Bonding Review Worksheet, PDF, Ionic Bonding
Recommended for you
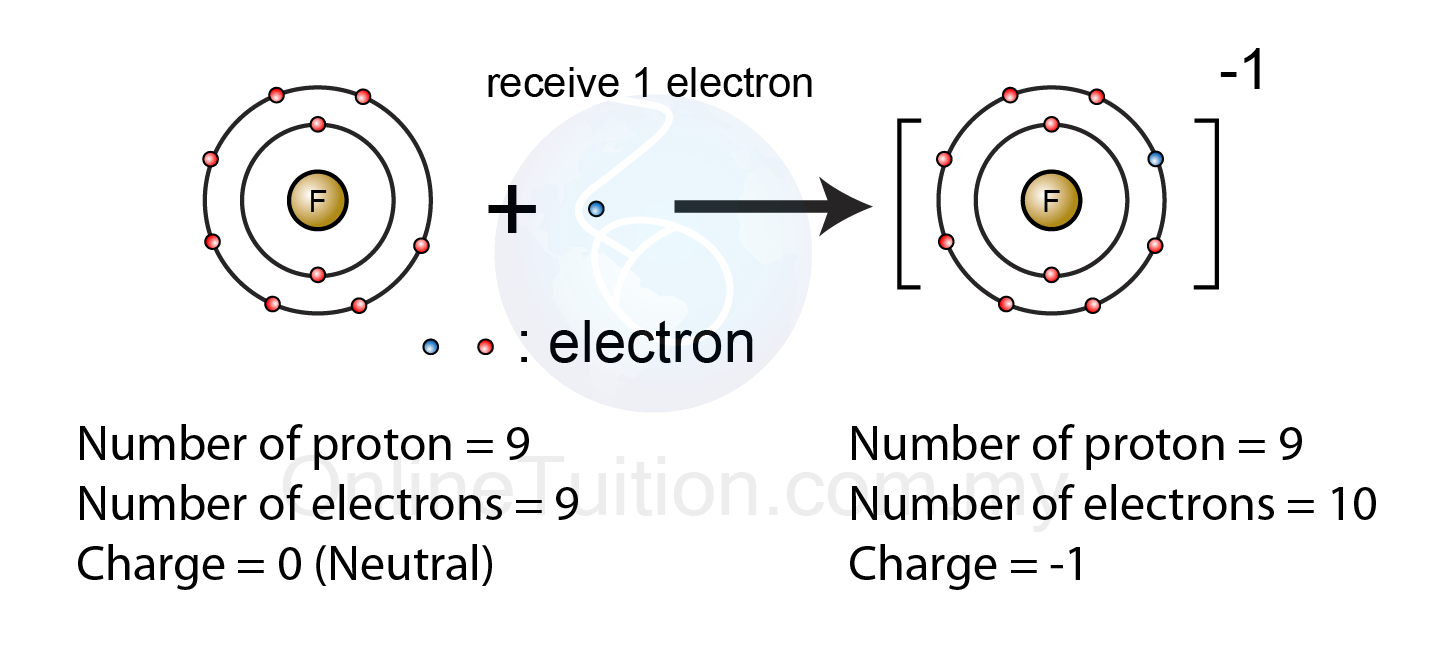 Formation of Negative Ions - SPM Chemistry14 Jul 2023
Formation of Negative Ions - SPM Chemistry14 Jul 2023 Negative Ion/Anti-5g Products Are Actually RADIOACTIVE14 Jul 2023
Negative Ion/Anti-5g Products Are Actually RADIOACTIVE14 Jul 2023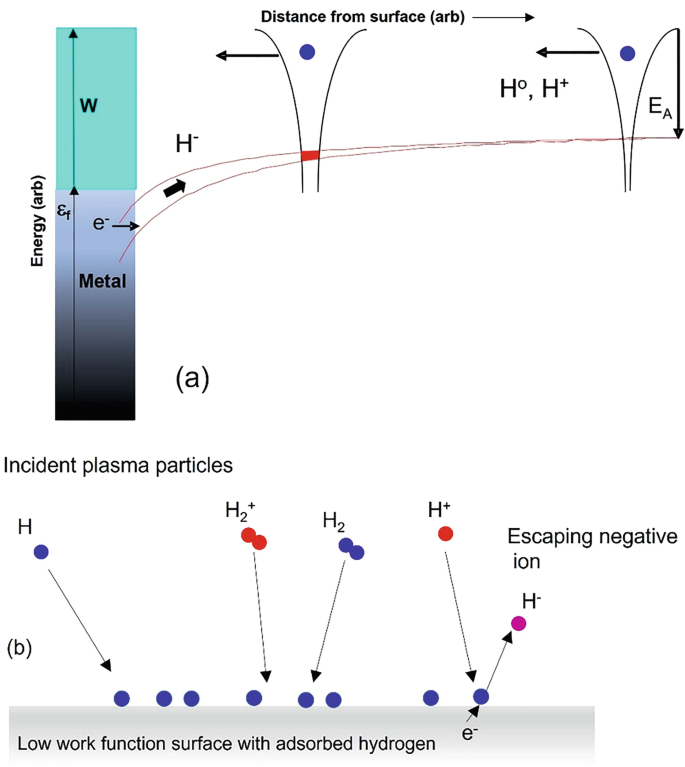 The Plasma Sheath in Negative Ion Sources14 Jul 2023
The Plasma Sheath in Negative Ion Sources14 Jul 2023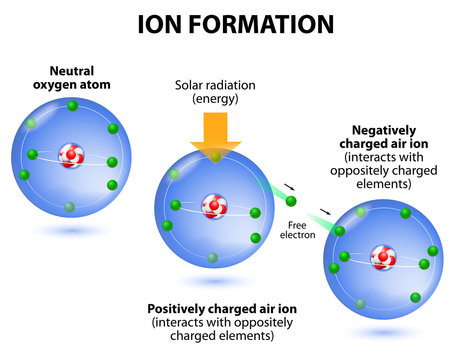 Negative Ions Images – Browse 32,510 Stock Photos, Vectors, and14 Jul 2023
Negative Ions Images – Browse 32,510 Stock Photos, Vectors, and14 Jul 2023 Drag each positive ion to bond it with a negative ion to form the14 Jul 2023
Drag each positive ion to bond it with a negative ion to form the14 Jul 2023- What is negative ion? - Negative Ion Apparels 负离子14 Jul 2023
- Bad News: Those Popular Negative Ion Bands Are Secretly Sending Radiation Into Your Body14 Jul 2023
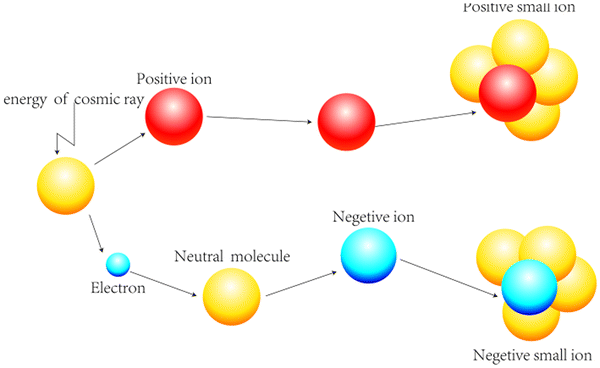 Generation and Determination of Negative Air Ions14 Jul 2023
Generation and Determination of Negative Air Ions14 Jul 2023 Electrodepot Negative Ion Generator - High Voltage ionizer 7.5Kv Plasma Module 110-120 VAC : Home & Kitchen14 Jul 2023
Electrodepot Negative Ion Generator - High Voltage ionizer 7.5Kv Plasma Module 110-120 VAC : Home & Kitchen14 Jul 2023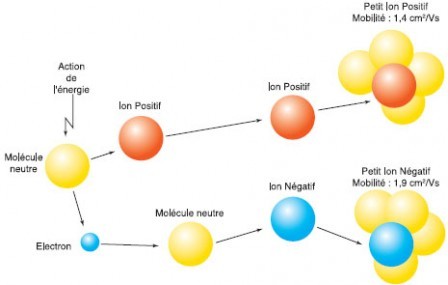 Are all negative ions the same ? - Ions négatifs14 Jul 2023
Are all negative ions the same ? - Ions négatifs14 Jul 2023
You may also like
 Roland Iten Belt buckles, Belt, Buckles14 Jul 2023
Roland Iten Belt buckles, Belt, Buckles14 Jul 2023 Esther Yellow Embroidered Tie Sleeve Corset Top – Lea Clothing Co.14 Jul 2023
Esther Yellow Embroidered Tie Sleeve Corset Top – Lea Clothing Co.14 Jul 2023 All In Motion Colorblock Athletic Leggings for Women14 Jul 2023
All In Motion Colorblock Athletic Leggings for Women14 Jul 2023 Anime Dragon Ball Son Goku Action Figure Toys Demoniacal Fit Df Shf Shining Soul Super Saiyan14 Jul 2023
Anime Dragon Ball Son Goku Action Figure Toys Demoniacal Fit Df Shf Shining Soul Super Saiyan14 Jul 2023 Women’s Warm Hiking Fleece - SH50014 Jul 2023
Women’s Warm Hiking Fleece - SH50014 Jul 2023 Class V Camp Print 5-Panel - The North Face14 Jul 2023
Class V Camp Print 5-Panel - The North Face14 Jul 2023 What Are the Causes of Extreme Fatigue in the Elderly?14 Jul 2023
What Are the Causes of Extreme Fatigue in the Elderly?14 Jul 2023 Women Ladies Heating Leggings USB Battery Rechargeable Winter Warm14 Jul 2023
Women Ladies Heating Leggings USB Battery Rechargeable Winter Warm14 Jul 2023 Celine 2021 Logo Sports Bra Crop Top - Yellow Tops, Clothing - CEL22880614 Jul 2023
Celine 2021 Logo Sports Bra Crop Top - Yellow Tops, Clothing - CEL22880614 Jul 2023 adviicd Yoga Pants For Women Casual Summer Yoga Women's Cross14 Jul 2023
adviicd Yoga Pants For Women Casual Summer Yoga Women's Cross14 Jul 2023
















