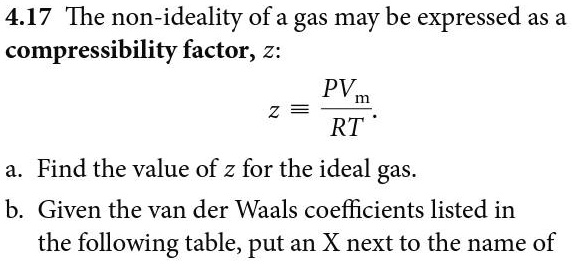What is the compressibility factor (Z) for 0.02 mole of a van der Waal
By A Mystery Man Writer
Last updated 20 Sept 2024

(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5
Filo Student Questions For CBSE , Grade 9
Energies, Free Full-Text
Unit 10 real gases vdw fl14 final
11111 Umu) 32 min 46. The ratio of van der Waals' constants a and
SOLVED: 4.17 The non-ideality of a gas may be expressed as a
Punjabi] The compressibillity of a gas is greater than unity at 1 atm
Real Gas - an overview
Deviation of real gases from ideal behaviour can be studied by plots o
The compressibility factor for nitrogen at 330 K and 800 atm is
What is the compressibility factor Z for 0.02 mole of a van der waal's gas at pressure of 0.1 atm. Assume the size of gas molecule is negligible. Given: RT =20 L
20 dm^(3) of SO(2) diffuse through a porous partitions in 60 second Wh
3.2 Real gas and compressibility factor – Introduction to
physical chemistry - Why do some gases have lower value of Z for a
Which gases behave least like ideal gases? - Quora
What is the compressibility factor (Z) for 0.02 mole of a van der
Recommended for you
 Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be14 Jul 2023
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be14 Jul 2023 Oil & Gas Softwares on X: Gas Compressibility Factor Calculator14 Jul 2023
Oil & Gas Softwares on X: Gas Compressibility Factor Calculator14 Jul 2023![PDF] Two Simple yet Accurate Equations for Calculating the](https://d3i71xaburhd42.cloudfront.net/01600927c4a2a03da177c4ee07cdbe81de887fc8/5-Figure3-1.png) PDF] Two Simple yet Accurate Equations for Calculating the14 Jul 2023
PDF] Two Simple yet Accurate Equations for Calculating the14 Jul 2023- Compressibility Factor Charts14 Jul 2023
 Virial coefficients: empirical approx. of the compression factor14 Jul 2023
Virial coefficients: empirical approx. of the compression factor14 Jul 2023- Real Gases vs Ideal Gases & the Compressibility Factor14 Jul 2023
 My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created14 Jul 2023
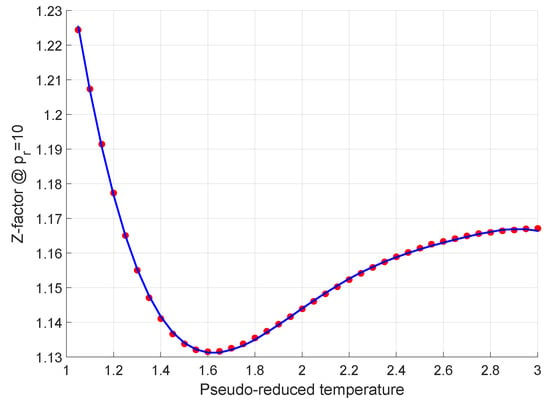
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created14 Jul 2023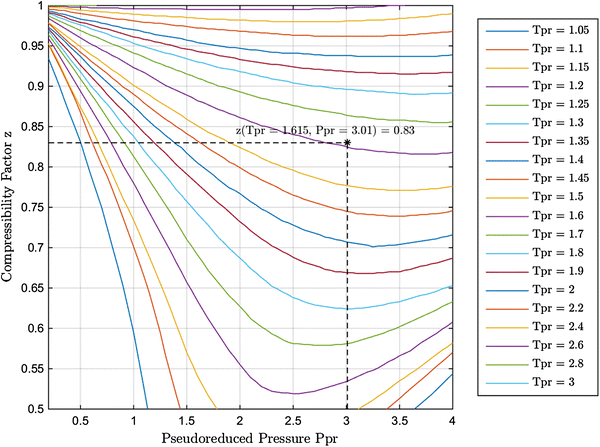 New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms14 Jul 2023
New explicit correlation for the compressibility factor of natural gas: linearized z-factor isotherms14 Jul 2023 Oil & Gas Softwares on X: Gas Compressibility Factor Calculator (Z-Factor) New App for #iPhone and #iPad #wellcontrol #drilling #Oil and #Gas #apps at / X14 Jul 2023
Oil & Gas Softwares on X: Gas Compressibility Factor Calculator (Z-Factor) New App for #iPhone and #iPad #wellcontrol #drilling #Oil and #Gas #apps at / X14 Jul 2023- Solved 3.91. The definition of compressibility factor Z, Eq14 Jul 2023
You may also like
 Yoga mat Yin 4mm - Exhale print14 Jul 2023
Yoga mat Yin 4mm - Exhale print14 Jul 2023 Free People Around the Clock Jogger!!! NWT!!!14 Jul 2023
Free People Around the Clock Jogger!!! NWT!!!14 Jul 2023) Buy BELLEVINO Women Lingerie Set Bra And Skirt With Thong Panty14 Jul 2023
Buy BELLEVINO Women Lingerie Set Bra And Skirt With Thong Panty14 Jul 2023 Apolla The Alpha Shock Half Sole Compression With Traction Dance14 Jul 2023
Apolla The Alpha Shock Half Sole Compression With Traction Dance14 Jul 2023- Leonisa Lace Side Seamless Thong Panty - Beige L14 Jul 2023
 3/6PCS LOT Womens Sexy Satin Bikini Panties Silky Brief Sexy Underwear S M L XL £13.21 - PicClick UK14 Jul 2023
3/6PCS LOT Womens Sexy Satin Bikini Panties Silky Brief Sexy Underwear S M L XL £13.21 - PicClick UK14 Jul 2023 Nike Sports bra SWOOSH in black/ white14 Jul 2023
Nike Sports bra SWOOSH in black/ white14 Jul 2023 Multicolor Floral Print Overcoat Styled Co-ord Set for Girls14 Jul 2023
Multicolor Floral Print Overcoat Styled Co-ord Set for Girls14 Jul 2023 MAGIC Bodyfashion - Silicone Bra Straps (15mm)14 Jul 2023
MAGIC Bodyfashion - Silicone Bra Straps (15mm)14 Jul 2023 Xiaodriceee See Through Underwear for Women Sheer Kuwait14 Jul 2023
Xiaodriceee See Through Underwear for Women Sheer Kuwait14 Jul 2023