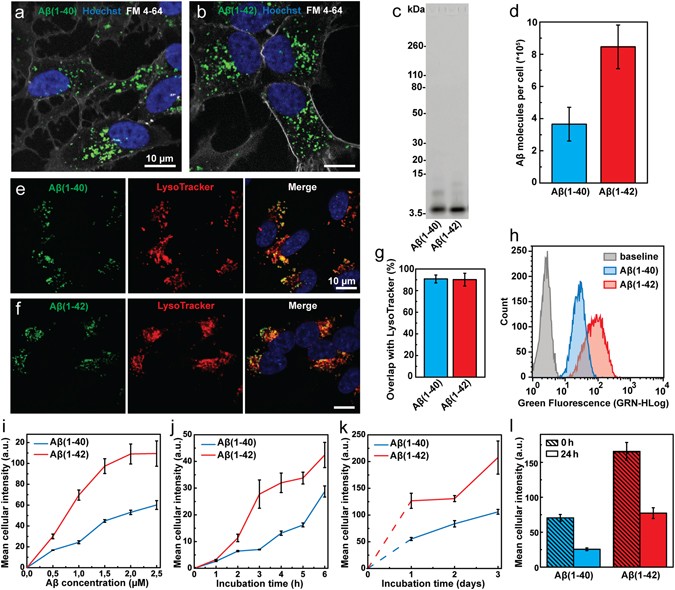
Endocytic uptake of monomeric amyloid-β peptides is clathrin- and dynamin-independent and results in selective accumulation of Aβ(1–42) compared to Aβ(1–40)
By A Mystery Man Writer
Last updated 19 Sept 2024
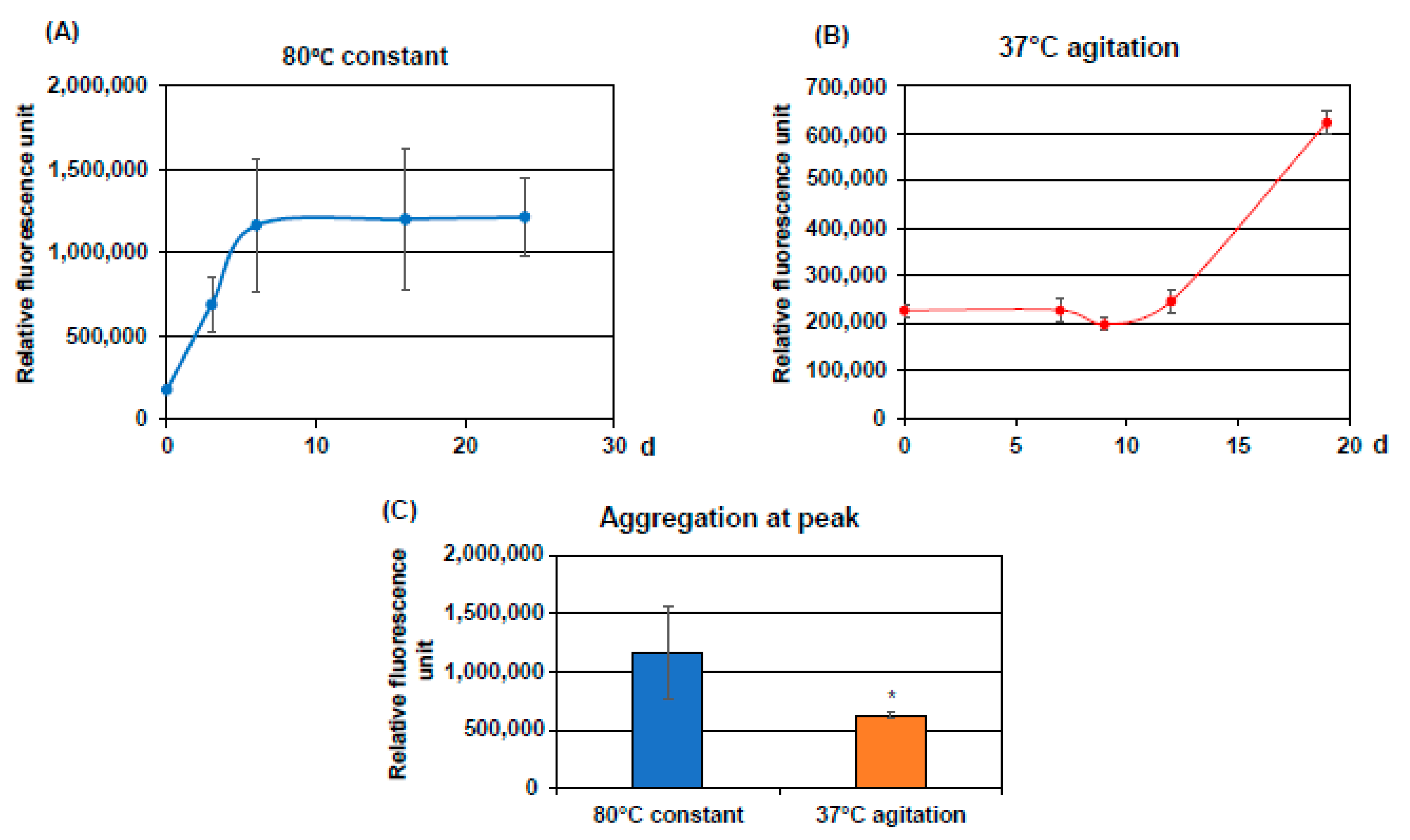
Designed Cell-Penetrating Peptide Inhibitors of Amyloid-beta Aggregation and Cytotoxicity - ScienceDirect
Designed Cell-Penetrating Peptide Inhibitors of Amyloid-beta Aggregation and Cytotoxicity - ScienceDirect
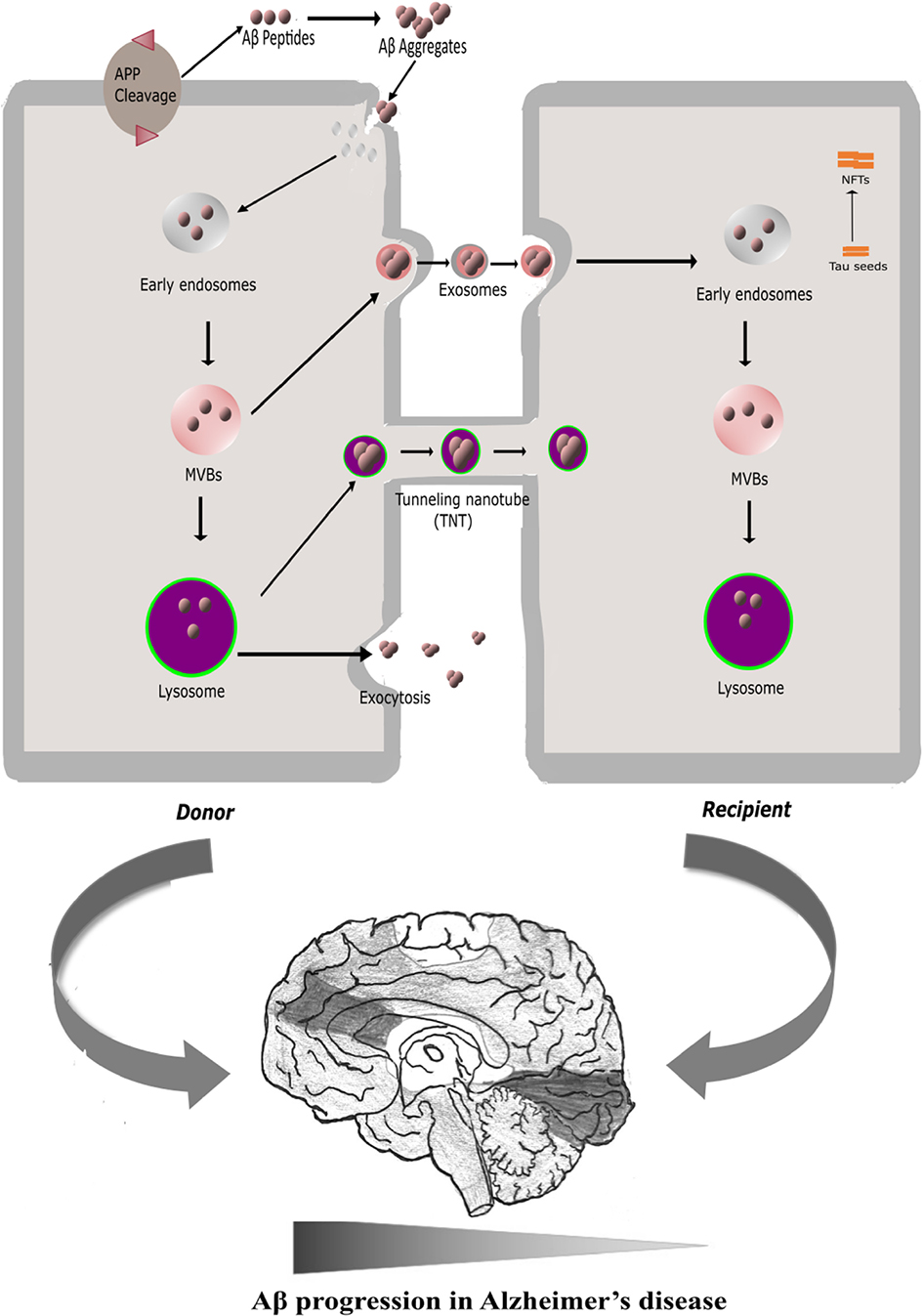
Misfolded amyloid-β-42 impairs the endosomal–lysosomal pathway
Endocytic pathways mediating oligomeric Aβ42 neurotoxicity, Molecular Neurodegeneration
The amyloid-β degradation intermediate Aβ34 is pericyte-associated and reduced in brain capillaries of patients with Alzheimer's disease, Acta Neuropathologica Communications
Frontiers Membrane interaction to intercellular spread of pathology in Alzheimer's disease
IJMS, Free Full-Text
IJMS, Free Full-Text
Evidence for aggregation-independent, PrPC-mediated Aβ cellular internalization. - Abstract - Europe PMC
Amyloid-beta peptides 40 and 42 employ distinct molecular pathways for cell entry and intracellular transit at the BBB endothelium
IJMS, Free Full-Text
Recommended for you
 ROXs 42Bb: Astronomers May Have Discovered New Class of Planets, Astronomy14 Jul 2023
ROXs 42Bb: Astronomers May Have Discovered New Class of Planets, Astronomy14 Jul 2023 NAV B-CHRONO 42 green Ltd. Edition14 Jul 2023
NAV B-CHRONO 42 green Ltd. Edition14 Jul 2023 Laco Pilot Aachen 42 B-Type Automático 861690.2 - Corvus14 Jul 2023
Laco Pilot Aachen 42 B-Type Automático 861690.2 - Corvus14 Jul 2023 Ruta 42-B on X: A todos nuestros usuarios les recordamos que seguimos brindándoles el servicio de 4:00 a.m. a 7:30 p.m. #QuedateEnCasa / X14 Jul 2023
Ruta 42-B on X: A todos nuestros usuarios les recordamos que seguimos brindándoles el servicio de 4:00 a.m. a 7:30 p.m. #QuedateEnCasa / X14 Jul 2023 fortis novonaut n-42 the ultimate space watch14 Jul 2023
fortis novonaut n-42 the ultimate space watch14 Jul 2023 Ruta 42-B on X: That booty! <3 #coaster #42B / X14 Jul 2023
Ruta 42-B on X: That booty! <3 #coaster #42B / X14 Jul 2023 PSAT Scoring: How Does It Work?14 Jul 2023
PSAT Scoring: How Does It Work?14 Jul 2023 AO/OTA classification of tibial diaphyseal fractures. 914 Jul 2023
AO/OTA classification of tibial diaphyseal fractures. 914 Jul 2023- Lenceria M - Luce hermosa, luce lencería M 👙 Brasier 42B $120 La belleza está en tu interior 🥰14 Jul 2023
 42-B - URKO MUSICAL14 Jul 2023
42-B - URKO MUSICAL14 Jul 2023
You may also like
- Beauty Salon, Nail Bar - Campden Beauty - Chipping Campden, England14 Jul 2023
 LADIES SKELETON LEGGINGS PLUS SIZE HALLOWEEN FANCY DRESS COSTUME14 Jul 2023
LADIES SKELETON LEGGINGS PLUS SIZE HALLOWEEN FANCY DRESS COSTUME14 Jul 2023![Twisted Series by Ana Huang [Twisted Love; Twisted Games; Twisted Hate and Twisted Lies]](https://m.media-amazon.com/images/I/71FwbwJ6LJL._AC_UF1000,1000_QL80_.jpg) Twisted Series by Ana Huang [Twisted Love; Twisted Games; Twisted Hate and Twisted Lies]14 Jul 2023
Twisted Series by Ana Huang [Twisted Love; Twisted Games; Twisted Hate and Twisted Lies]14 Jul 2023 Calça Jogger Jeans com Amarração no Cós e Detalhes Puídos Azul14 Jul 2023
Calça Jogger Jeans com Amarração no Cós e Detalhes Puídos Azul14 Jul 2023 Bali Passion for Comfort Minimizer Underwire Bra, Silver Lace, 38DD at Women's Clothing store14 Jul 2023
Bali Passion for Comfort Minimizer Underwire Bra, Silver Lace, 38DD at Women's Clothing store14 Jul 2023 SPOKOFIT All Purpose 1/4-Inch High Density Anti-Tear Exercise Yoga14 Jul 2023
SPOKOFIT All Purpose 1/4-Inch High Density Anti-Tear Exercise Yoga14 Jul 2023 Used Kuhl Trekr Pants14 Jul 2023
Used Kuhl Trekr Pants14 Jul 2023 Aluminum Geodesic Domes Phillips Tank & Structure14 Jul 2023
Aluminum Geodesic Domes Phillips Tank & Structure14 Jul 2023 14K Gold Inlay Men's Tungsten Wedding Band – RING BEAR14 Jul 2023
14K Gold Inlay Men's Tungsten Wedding Band – RING BEAR14 Jul 2023 A+ Essential Metal Snap Hook Sample Kit14 Jul 2023
A+ Essential Metal Snap Hook Sample Kit14 Jul 2023