physical chemistry - Is the compressibility factor smaller or
By A Mystery Man Writer
Last updated 21 Sept 2024

The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate.
In
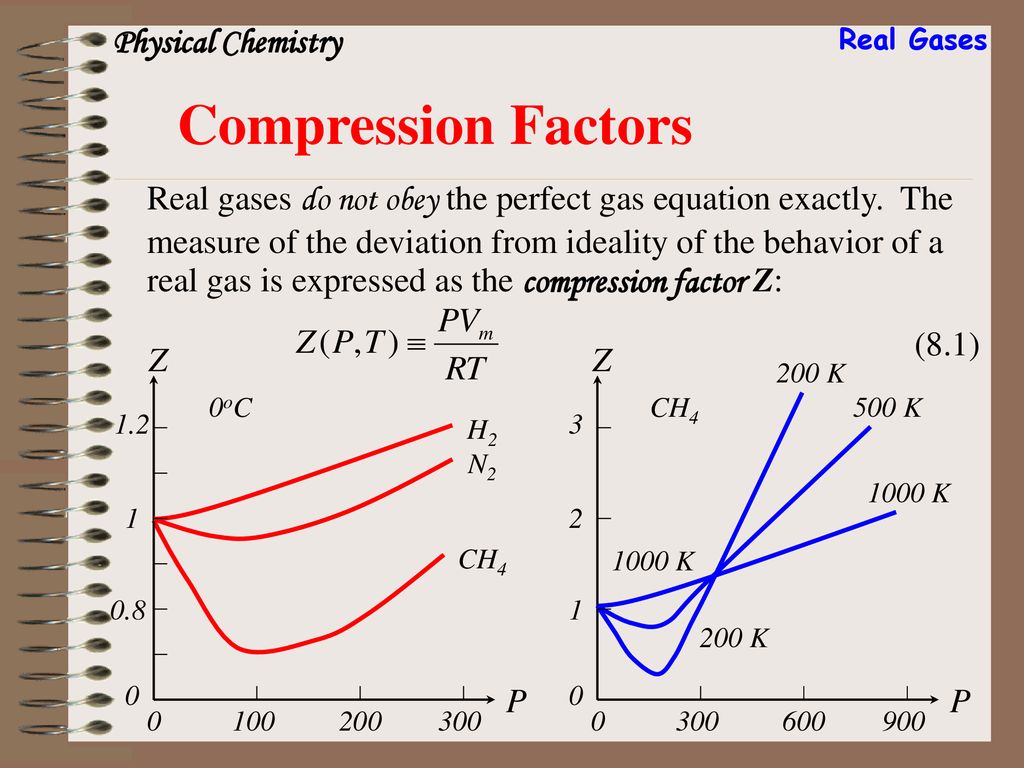
Chapter 8 Real Gases. - ppt download
11.3: Critical Phenomena - Chemistry LibreTexts
Compressibility Factor of Gas Overview, Equation & Chart
Acentric Factor - an overview
physical chemistry - Is the compressibility factor smaller or
At Critical Temperature,pressure and volume . The compressibility
Physical Chemistry The Compression Factor (Z) [w/1 example
3.2 Real gas and compressibility factor – Introduction to
The compressibility factor `(Z)` of real gas is usually less than
What Exactly is The Compressibility of Fluids?
compressibility Factor v/s Pressure/ Temperature Graph . States of
ASTM D3588-98 - Standard Practice for Calculating Heat Value, Compressibility Factor, and Relative Density of Gaseous Fuels
Compressibility factor - Wikipedia
Determine Compressibility of Gases
Recommended for you
 Compressibility Factor of Gas, Overview, Equation & Chart - Lesson14 Jul 2023
Compressibility Factor of Gas, Overview, Equation & Chart - Lesson14 Jul 2023 Compressibility factor (Z) for a van der Waals real gas at critical point is14 Jul 2023
Compressibility factor (Z) for a van der Waals real gas at critical point is14 Jul 2023 Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT14 Jul 2023
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT14 Jul 2023- At Critical Temperature,pressure and volume . The compressibility14 Jul 2023
 Real gasses For an ideal gas, the compressibility factor Z = PV14 Jul 2023
Real gasses For an ideal gas, the compressibility factor Z = PV14 Jul 2023 Chapter 3 - Physical Properties of Fluids: Gas Compressibility14 Jul 2023
Chapter 3 - Physical Properties of Fluids: Gas Compressibility14 Jul 2023 Objectives_template14 Jul 2023
Objectives_template14 Jul 2023 My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created14 Jul 2023
My publications - CHM 201-LECTURE IV-REAL GASES - Page 8 - Created14 Jul 2023 Calculate the Compressibility Factor 'z' for Hydrocarbon Gases • zFactor14 Jul 2023
Calculate the Compressibility Factor 'z' for Hydrocarbon Gases • zFactor14 Jul 2023 For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may14 Jul 2023
For a given gas, a graph is shown between compressibility factor (Z) and Pressure (P).Select the incorrect statement(s) about the various temperature relations.a)Temperature T1 must be above critical temperature (TC).b)Temperature T2 may14 Jul 2023
You may also like
 Leg Warmers, Capezio, 12 Legwarmer Black , $11.00, from VEdance LLC, The very best in ballroom and Latin dance shoes and dancewear.14 Jul 2023
Leg Warmers, Capezio, 12 Legwarmer Black , $11.00, from VEdance LLC, The very best in ballroom and Latin dance shoes and dancewear.14 Jul 2023 Skechers Uno Gen1 Trainers Junior Air Bubble Trainers Memory Foam Shoes14 Jul 2023
Skechers Uno Gen1 Trainers Junior Air Bubble Trainers Memory Foam Shoes14 Jul 2023 Adidas Women's Cross Back Sports Bra XS Black White Light Support New Retail $4014 Jul 2023
Adidas Women's Cross Back Sports Bra XS Black White Light Support New Retail $4014 Jul 2023 Pink Two Piece Jumpsuit - Wide-Leg Pant Set - Two-Piece Pant Set - Lulus14 Jul 2023
Pink Two Piece Jumpsuit - Wide-Leg Pant Set - Two-Piece Pant Set - Lulus14 Jul 2023 Non Slip Fur Lined Thermal Snow Boots Slip Wear Resistance - Temu14 Jul 2023
Non Slip Fur Lined Thermal Snow Boots Slip Wear Resistance - Temu14 Jul 2023 Powr Labs Bluetooth Heart Rate Monitor Armband - ANT14 Jul 2023
Powr Labs Bluetooth Heart Rate Monitor Armband - ANT14 Jul 2023 Obsession Red Verbena (Verbena 'Obsession Red') in Brainerd Baxter14 Jul 2023
Obsession Red Verbena (Verbena 'Obsession Red') in Brainerd Baxter14 Jul 2023 Suddenly shops are full of retro undies including Marilyn Monroe's14 Jul 2023
Suddenly shops are full of retro undies including Marilyn Monroe's14 Jul 2023 Talk to explore history of Black jazz musicians in Eastern Canada – The Brock News14 Jul 2023
Talk to explore history of Black jazz musicians in Eastern Canada – The Brock News14 Jul 2023- Smooth Seamless Thong *3 Pack14 Jul 2023